Recent Advances on New group V graphyne
Recently, Prof. Hao Zhang and Prof. Rongjun Zhang from the Department of Optical Science and Engineering of the School of Information Science and Technology, in collaboration with Prof. Wenjun Liu from the School of Microelectronics, designed new group-V graphyne and investigated their properties and thermoelectric properties. On March 20, this work was published on Materials Today Physics entitled New group V graphyne: two-dimensional direct semiconductors with remarkable carrier mobilities, thermoelectric performance, and thermal stability.
Carbon is an active element with rich valence states, and the inventions of its allotropes, e.g. fullerene, carbon nanotubes, carbon fiber and graphene, have triggered significant impacts on industries and scientific researches for decades. Graphyne is a new allotrope of carbon, which was originally predicted by R. H. Baughman in 1987. Graphyne consists of sp and sp2 hybrid-bonding carbon atoms, and it is the most stable among the artificially designed carbon allotropes currently. It has been reported that, graphyne possesses high carrier mobility and low lattice thermal conductivity, which may lead to wide applications in the fields of energy storage and transformation, biomedicine, and seawater desalination. In 2010, graphyne was successfully fabricated, the research is speeding up in recent years.
However, similar to graphene, graphyne possesses a Dirac cone-shaped electronic structure, and thus the on-off ratio of graphyne-based FET device is low, which limits its application in the field of nanoelectronics. To deal with the gapless bandstructure of graphyne, in this work, we replaced the four carbon atoms in the graphene with five group elements of N, P, As, and in this way, we successfully designed three new kinds of graphyne derivatives, i.e. C16N4, C16P4, C16As4. We investigated the thermo-dynamical stabilities of these new materials, and found that they maintain stable up to high temperature of 1500 K, which means they are believed to be easily fabricated.
By calculations, we show that they are direct-band-gap semiconductors, and amazingly, we found that, their electron mobilities reach the order of 105cm2V-1s-1, which is comparable to graphene, revealing their wonderful potential in the microelectronic devices.
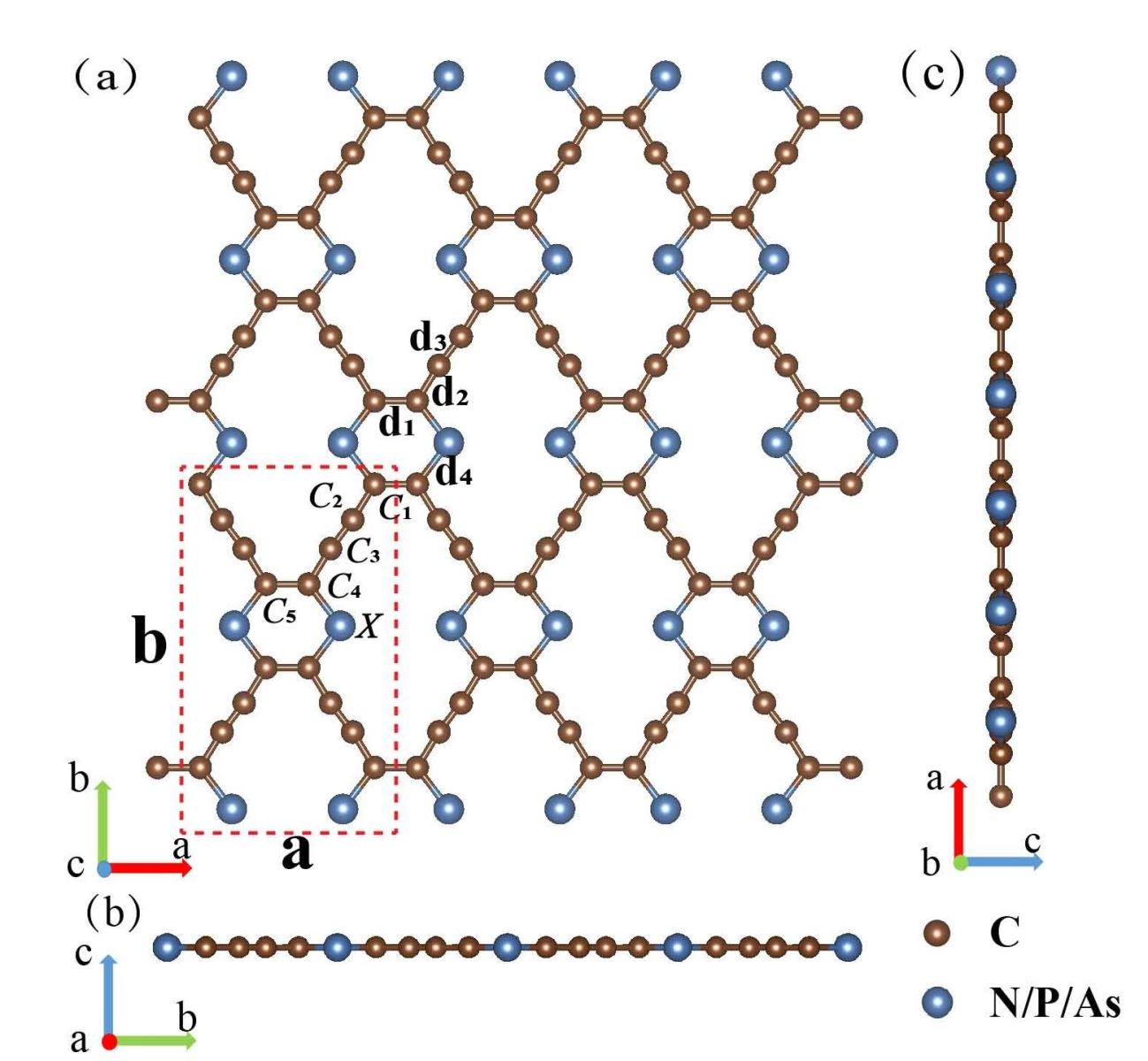
Figure 1 Crystal structure of group V graphyne
The complex crystal structure and the introduction of Group V elements further reduce the lattice thermal conductivity of the material, which is three orders of magnitude smaller than graphene at room temperature. This means that these materials are expected to convert more thermal energy into electrical energy with high efficiencies. We calculated the room temperature thermoelectric figure of merit (zT value), which is generally used to determine the energy-conversion efficiency of thermoelectric materials, and we found the zT values of the new graphyne derivative are in the range of 0.62-0.69, which is comparable to traditional thermoelectric materials.
The achievements we obtained in this work will lay the foundation for the futural application of graphyne-derivative materials in nanoelectronic devices, micro-nano energy devices, etc. This work is the result of cooperative researches of multiple departments and multiple research groups of Fudan University. Wu Yu, a PhD student in the Department of Optical Science and Engineering, Ma Congcong, a 2018 PhD student in the Department of Electric Light Sources, and Chen Ying, a 2018 graduate student of the Institute of Engineering and Applied Technology, are the co-first authors. This work was supported by the National Natural Science Foundation of China (No: 11374063, 11674062, 11544008), Shanghai Natural Science Foundation (19ZR1402900, 18ZR1402500), etc.
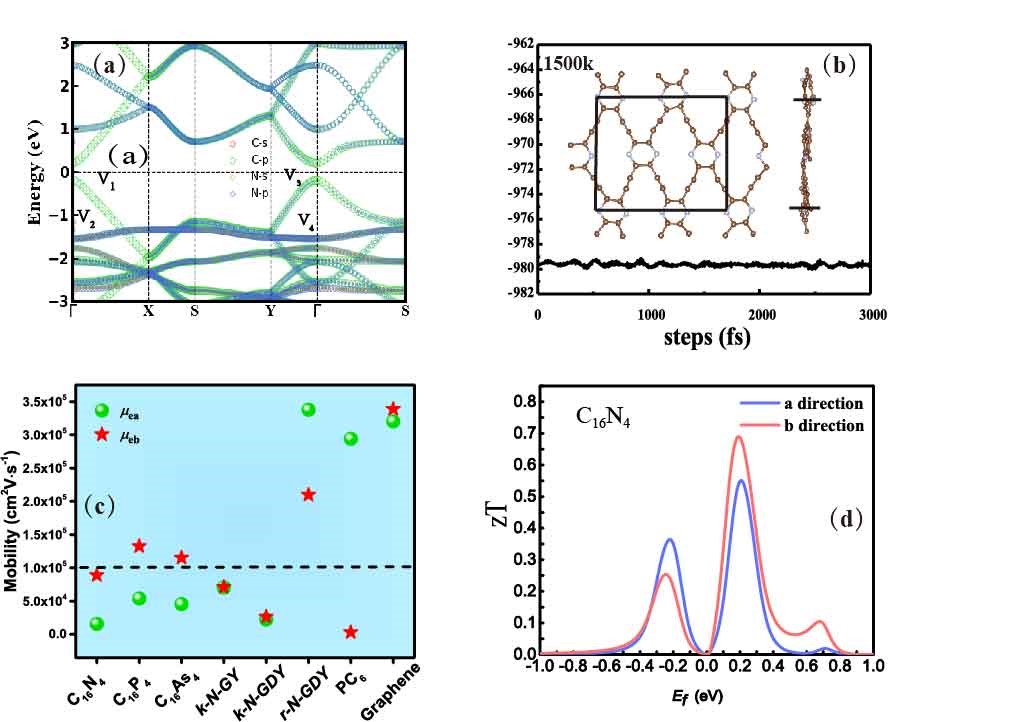
Figure 2 a) Band structure of C16N4 b) crystal structure of C16N4 at 1500 K under AMID c) comparison of electron mobilities of the three predicted materials with carbon-based materials with high mobility d) room temperature zT value of C16N4

